Comparison of possible upstream
promoter elements that determine alternative splicing in human, rat, and mouse
A1 adenosine receptors
Adrienne J. Williams, Nguyen D. Khoa, Dave Delano, Bruce N. Cronstein.
New York University School of Medicine, New York, NY
Background
Adenosine is a nucleoside released from tissues in response to metabolic stress or damage that was found in 1970 to regulate cell function by occupying specific receptors on its surface.1 These 7-transmembrane members of the G-protein coupled receptor superfamily have been found to be involved in many physiological responses, making them interesting targets for pharmaceutical therapy of diseases ranging from neurodegenerative and chronic inflammatory disorders to several pathogenic diseases.2,3
There are four subtypes of adenosine receptor - A1, A2A, A2B, and A3.4 Each receptor subtype has distinct ligand binding properties and different patterns of tissue expression. All four have been cloned from a variety of species and had their gene structure repeated. Of particular interest is the A1AR subtype, which has been found to contain six exons, although only the last four have been found in mature mRNAs. Furthermore, human tissue can express a combination of either exons 3, 5, and 6 or exons 4, 5, and 6.5 This alternative splicing has been found to be due to dual promoter elements located upstream of exons 3 and 4. The promoters, A and B, are separated by an exon, and when one set of upstream elements are stimulated, the other promoter is inactive. So far, GRE, AP1, AP2, NfKB and SRE sites have been found to be involved in activation of promoter A or B for human A1 adenosine receptor depending on the agent involved.6 (Appendix A: Fig.1)
My research with adenosine receptors focuses on the antagonistic properties of the A1 and A2A subtypes in human dermal fibroblasts treated with ligands to the receptor for advanced glycation endproducts (RAGE). Closer analysis of the dual promoters upstream of the A1 adenosine receptor’s coding sequence is of interest in helping to better understand potential venues of alternative splicing, which makes designing PCR primers for this receptor difficult. By comparing the A1AR upstream promoter elements of different species to a human one, it is hoped an idea of more promoter elements that are involved in splicing can be found.
Methods
Sequence Retrieval. Genomic sequences were retrieved through either a BLAST search for A1 adenosine receptor on NCBI’s Nucleotide server (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Nucleotide) or by browsing the genomic database of UCSC (http://genome.ucsc.edu/). For a cross-species comparison, BLAST sequences were selected that had at least 75 bp in common to human A1AR mRNA exons (NM_000674). (Appendix B: a1rdnaseq5, a1rdnaseq2) More thorough genetic analysis was limited to species with highly researched genomes that were able to provide introns, namely human, mouse, and rat. The introns needed to determine upstream promoters were acquired using software at the UCSC database. About 1000bp of introns upstream of exons 3, 4, and 5 were initially selected as well as an area spanning 1000bp upstream of exons 3 and 4 and 350bp after, which will be referred to as the dual, or AB, promoter region of alternative splicing.
Multiple Alignment. Sequences were aligned using the program PILEUP and some were checked against each other using other alignment programs, BESTFIT and GAP, all provided by the NYU School of Medicine’s RCR. PILEUP was used to align all related A1 adenosine receptor sequences found initially by BLAST as well as the upstream promoter regions of exons 3, 4, 5 and the AB region for rat, mouse, and human A1AR. BESTFIT and GAP were performed on the upstream promoter regions of exons 4 and 5 but not on the AB promoter region due to time constraints.
Upstream Regulatory Elements and Promoter Prediction. Potential upstream regulatory elements were analyzed using TfSiteScan (http://www.ifti.org ). Because the program only optimally examines around 500 nucelotides at a time, sequences within promoter regions for AB and upstream of exons 3, 4, and 5 were selected based on the most PILEUP alignment between species, leading to reduced sequences of about 600 nucleotides in size. Promoters upstream of exons 3, 4, and 5 were processed but not fully analyzed due to time constraints. Promoter regions were predicted using the Neural Network Promoter Prediction program (http://www.fruitfly.org/seq_tools/promoter.html ), using the same 600 nucleotide sequence mentioned above for the AB and upstream 3, 4, and 5 promoter sequences. Results were then compared by hand across species for areas of similarity for the AB promoter region but not the others, again due to time constraints.
Results
A1 adenosine receptor cDNA was found for 37 different sequences using the BLAST search, seven of those from different species. (Appendix C: a1rdnaseq10, a1rdnaseq, a1rdnaseq4). The sequences were then aligned together mostly to try out PILEUP, but to also see what species had enough conservation with human A1AR to include exons 3 and 4, the area of alternative splicing. (Appendix D: AB_001089_pileup.htm) The most conserved regions not surprisingly included exons 5 and 6, the coding region for the receptor (PILEUP positions 450-1350). Out of the 37 sequences, 22 had homology similar to the A1 adenosine receptor’s 3rd or 4th exon (PILEUP positions 200-450). Of those, seven were human (NM_000674, BC_026340, X68485, S45235, AC_105940, L22214, S56143), six were murine (AK_043954, AK_0457229, AK_038774, AF_133099, U05671, XM_129465), four were rat (M64299, M69045, NM_017155, Y12519), two were bovine (X63592, M86261) and the rest were canine (X14051), guinea pig (U04279), and rabbit (L01700). Only three of these seven species were determined after research to have sufficiently developed genomes to explore the introns surrounding these exons – human, mouse, and rat.
The AB promoter regions of human and mouse A1 adenosine receptors have been previously studied6,7 and were easily retrieved from the UCSC database with the introns intact. Murine and rat A1AR cDNA have no equivalency to the third exon, so additional upstream nucleotides were included for comparative purposes. It should also be noted that the rat genome has not been as extensively sequenced as the other two species for this chromosomal area, and therefore a portion of the rat promoter area farthest upstream from the exons has yet to be discovered. An approximation of the rat promoter region was determined based on its high sequence homology to mouse DNA. (Appendix E: abpromoters) As mentioned in Methods, the selected promoter region determined from PILEUP covers 1000bp upstream of human exons 3 and 4 with an additional 350 bp downstream. A general curiosity to see if there were any common promoters upstream of human A1 adenosine receptor exons 3, 4, and 5 led to retrieving sequences 1000bp upstream from each of the three species for these sequences as well. (Appendix F: upstream-promoters)
Alignment of the ~827 bp sequences upstream of exon 3 found about 250 bp of homology (~550-750) for two of the species, human and mouse. (Appendix G: fragment_hum_pileup_149872.htm) The rat upstream sequence was too underdeveloped to be particularly effective. The poor upstream development of this sequence in both mouse and rat eventually led to pursuit of upstream exon 3 being dropped. Alignment of the ~800 bp sequences upstream of exon 4 found about 275 bp of homology (~300-575) for all three species. (Appendix H: human_upstre_pileup_37541.htm) This was confirmed with two other alignment programs, BESTFIT and GAP, which compare two sequences at a time. (Appendix I: rat_upstream_gap_118742.htm, human_upstre_gap_118716.htm, human_upstre_gap_118653.htm, rat_upstream_bestfi_119096.htm, human_upstre_bestfi_119051.htm, human_upstre_bestfi_117897.htm) Alignment of the ~873 bp sequences upstream of exon 5 found about 130 bp of homology (~360-490) for all three species, with another possible 100bp that the rat introns did not have available, but may likely have been there due to the strong homology between mouse and rat genomes. (Appendix J: human_upstre_pileup_37213.htm) BESTFIT and GAP confirmed this alignment. (Appendix K: rat_upstream_gap_119005.htm, human_upstre_gap_118747.htm, human_upstre_gap_118785.htm, rat_upstream_bestfi_119230.htm, human_upstre_bestfi_119158.htm, human_upstre_bestfi_114017.htm)
Alignment of the main sequence of interest, the AB promoter region (~2107bp total), found about 580 bp of sequence homology (~940-1527) for all three species. (Appendix L: human_promot_pileup_163162.htm, Fig. 2) Compared to the promoter region in Fig. 1, this aligned area is 50bp after the AP2 promoter element of promoter A and ends about 7bp after Promoter B.6 From the experience of analyzing GAP and BESTFIT on the previous sequences and due to time constraints, it was not considered necessary for these alignments to be further confirmed. As a result of PILEUP, all sequences retrieved were trimmed down to the areas they most aligned with for better promoter analysis to at least 700 bp. (Appendix M: upstream promoters aligned, abpromotersaligned) There is a slight discrepancy between the human and rodent DNA sequences due to a 600 bp separation between the dual promoters in humans while only a 250 bp gap in the mice and rats.6,7 As a result, the alignment of the three sequences shown above takes place after Promoter A in human A1AR.
The promoter prediction software found two possible start sites upstream of human exon 4 and six upstream of the rat and mouse equivalents. However, while the rat and mouse sites are almost identical, as would be expected, neither has a start site in common with the human ones. Two start sites were also found upstream exon 5 in the human sequence, but 4 with rat and only one with mouse. Surprisingly, there is not as much homology between rat and mouse here, and the rat and human sequences have one potential promoter start site in common (AGCCGGAGA in human compared to AGCCTGGAGA in rat). The score cutoff for these tests was 0.5. Earlier work was done on unaligned data that is also presented here. (Appendix N: neuralnetworkdataaligned, neuralnetworkdata) The AB promoter data used a score cutoff of 0.60. Four possible promoter start sites were found for its human sequence, nine in the mouse and seven in the rat. The human and mouse sequences had some promoter start sites in common (CGCCCCTCC for both, GTCACTCAGC for both, CGTACCATGT in human compared to ACACCATGT in mouse and rat), especially that last sequence, which matched all three species. The other start sites may also be as significant due to the chunks of information missing from the rat sequence. These areas translate to positions on the aligned human A1 adenosine receptor sequence from Fig. 2 as 949-950 bp, 960-980 bp, and 1500-1510 bp. These in turn translate to at least one previously determined start site as seen in Fig. 1 for exon 1B. (Appendix O: neuralnetworkdataab)
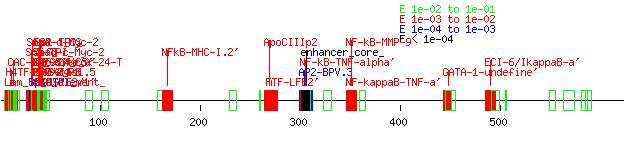
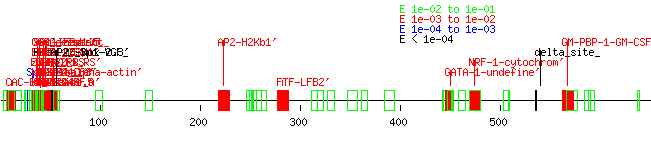
The search for promoter elements upstream of human exon 4 using Tfsitescan yielded many possibilities for it and its rat and mouse counterparts but very few similarities between all three, although the rat and mouse sequences, again, had a high level of homology between each other. (Appendix P: tfsitescandata4, human4, rat4, mouse4) There were even fewer matches upstream of exon five. (Appendix Q: tfsitescan data, human 5, rat5, mouse5) A truly thorough analysis of the data was not performed due to time constraints, although one GATA-1 site stood out at the exon 4 area between all three species. Earlier work with unaligned upstream exon 3 data showed how quickly I learned the value of tightly aligned data rather than just stuffing sequences into the software input. (Appendix R: tfsitescandata3, human3, rat3, mouse3) A closer inspection of the data was performed on the dual ab promoter region. (Appendix S: tfsitescandataab, humanab, mouseab, ratab, Fig. 3) This was done by examining the data for mouse and rat first, noting similar upstream promoter elements between them, and then seeing if those elements appeared in the human sequence. Areas that did have consensus between species included an SP-1 rich area after Promoter A, as well as several AP2, GCF, and GKCF sites and a FTF-LFB2’ and GATA-4 site. While the rat promoter region failed to show the SP_1-rich area, it is likely this is due to the early truncation of the sequence due to its genome not being completely finished. Elements in this area were found previously for both human and murine A1 adenosine receptors (NFkB, AP1, AP2, SRE1, SRE2, and GRE for humans; GATA-4 and Nkx2.5 for mice).6,7 Interestingly, many of these particular sites were not found to be similar across species.
Conclusion
So, ultimately I found an SP-1 rich area, as well as several AP2, GCF, GKCF and a FTF-LFB2, GATA-4 and undefined GATA-1 site in the dual promoter region of the A1 adenosine receptor in humans. I also located three transcriptional start sites, all due to comparing the genomic sequences from three different species. If I wanted to explore this further, I could wait for the genomes of more species to be further realized and widely available to further confirm my results. Working with scraps of the rat genome showed me how problematic a lack of information can be – fortunately there was enough data on another rodent, the mouse, to do some comparative analysis. I would also want to examine the data more on promoters directly upstream of exons 3, 4, and 5. These are the classic sites to design primers for the A1AR, the initial reason I took this project, although knowledge of the dual promoter alternative splice site is also of great importance. Finally, I suppose this method of cross-species comparison is faulty in that many potential promoter elements and transcription start sites, can be overlooked, as shown by my failure to pick up previously discovered promoter elements on human A1 adenosine receptor.6
References
1.Sattin, A, TW Rall. 1970. The effect of adenosine and adenosine nucleotides on the cyclic adenosine-3',5' phosphate content of guinea pig cerebral cortex slices. Mol Pharmacol. 6(1):13-23.
2. JW Daly. 1982. Adenosine receptors: targets for future drugs. J Med Chem 25: 197-207.
3. Poulson SA, RJ Quinn. 1998. Adenosine receptors: new opportunities for future drugs. Bioorg Med Chem. 6: 619-641.
4. Fredholm, BB, Abbrachio MP, Burnstock G et al. 1994. Nomenclature and classification of purinoceptors. Pharmacol Rev. 46(2):143-56.
5. Ren, H, GL Stiles.1994. Posttranscriptional mRNA processing as a mechanism for regulation of human A1 adenosine receptor expression. PNAS. 91(11):4864-4866.
6. Ren, H, GL Stiles.1999. Dexamethasone stimulates human A1 adenosine receptor (A1AR) gene expression through multiple regulatory sites in promoter B. Mol Pharmacol. 55(2):309-316.
7. Rivkees, SA, Kulkami, J, Browne, J, and Z Zhao. 1999. Characterization of the murine A1 adenosine receptor promoter, potent regulation by GATA-4 and Nkx.2.5. J Biol Chem. 274(20): 14204-14209.
Figures

FIGURE 1. A Human A1AR gene promoters and their flanking sequences. The transcription start sites for promoters A and B are labeled +1 and they are about 600 bp apart. The transcriptional factor-binding sites are underlined. The arrows pointing upward or downward indicate the boundaries of plasmid inserts for PmtA/-897 or PmtB/-422, respectively. The horizontal arrows indicate the boundaries of exons and intron. B The simplified structure of human A1AR promoters and regulatory elements. From Ren H, Stiles GL. 1999. Dexamethasone Stimulates Human A1 Adenosine Receptor (A1AR) Gene Expression through Multiple Regulatory Sites in Promoter B. Mol Pharmacol 55(2):309-316.

A
B
FIGURE 2. A Sequence alignment of the A1AR dual promoter region upstream of the human exons three and four compared to similar regions in mouse and rat genomic sequences. Capital letters indicate an exon; note that mouse and rat A1adenosine receptor sequences do not have this exon. To compare with Figure 1, the alignment of all three sequences starts about 50 bp after the AP2 upstream promoter element after Promoter A. The consensus ends about 7bp after the promoter region. Gap Creation Penalty: 5. Gap Extension Penalty: 1. Wavy lines indicate the end of a particular sequence; dots indicate an inserted gap. The original total length of the alignment was from 1 to 2101. B Multiple sequence alignment dendogram of the three species.
A
B
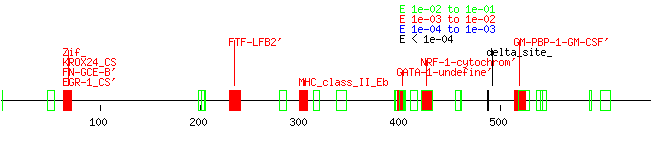
C
FIGURE 3. Upstream promoter elements as determined by TfSiteScan for A1 adenosine receptor in A human, B mouse and C rat. The aligned promoter regions from Figure 2 were used, on average about 624 bp in length. Upstream elements were listed in order by position and scored based on P-values for likelihood. Elements colored red, blue, and black are more likely to be possible promoter elements than green ones. The heavy cluster of elements in A and B at the first 100 bp indicate the SP-1 rich area; this is likely missing in C because those nucleotides are missing. Other elements of consensus between human and rodent are the FiTF-LFB2’, GATA-4, and GATA-1 sites. The murine and rat promoter elements have many similar elements due to their high homology in DNA sequences.